Biofilm Formation: From Infectious Diseases to Biotechnology
Historically, our understanding of bacterial physiology and development of antibiotics have been focused on planktonic (free-swimming) cells. However, the vast majority of bacteria in nature exist in surface-attached highly hydrated structures comprised of a polysaccharide matrix secreted by the bound bacterial cells, collectively known as biofilms (Figure below). With up to 1000 times higher tolerance to antibiotics and disinfectants compared to their planktonic counterparts, deleterious biofilms cause serious problems such as chronic infections in humans as well as persistent fouling and equipment failure in industry. Biofilms are blamed for billions of dollars of losses and more than 45,000 deaths annually in the U.S. alone. Despite the well-recognized significance of biofilms, the biofilm research is still in its infancy with many fundamental questions unanswered. Controlling biofilm is also an unmet challenge. With the efficacy of antibiotics and disinfectants being intrinsically limited, new approaches especially those with synergistic effects are desired.
|
The dynamic process of biofilm formation. The dark cells represent regular bacterial cells and the white cells represent persister cells. |
Research in modern microbiology has led to important discoveries that are shifting the paradigm of microbial control. For example, bacteria have been found to enter a dormant stage and form persister cells. Persister formation increases significantly in biofilms, allowing bacteria to survive the attack of both antimicrobials and host immune systems. Thus, persister cells play important roles in biofilm-associated antibiotic tolerance and facilitate the development of multidrug resistant bacteria (“superbug”). In addition, a cell-cell signaling system, named quorum sensing, has been identified in hundreds of bacterial species, which allows bacteria to synchronize gene expression by sensing and responding to cell density. Quorum sensing has been found to control biofilm development and persister formation in some bacterial species, e.g., Pseudomonas aeruginosa. We are inspired by these new discoveries and motivated by the urgent needs to improve the fundamental understanding of biofilm formation and develop better control methods.
Compared to deleterious biofilms that cause serious problems in both medical and engineering settings, biofilms of environmentally friendly bacteria have promising applications. Due to their intrinsic tolerance to toxic agents, such biofilms may provide promising solutions to currently unmet challenges such as the high cost in biofuel production due to low tolerance of microbes to the fermentation products and difficulties in bioremediation of toxic contaminants.
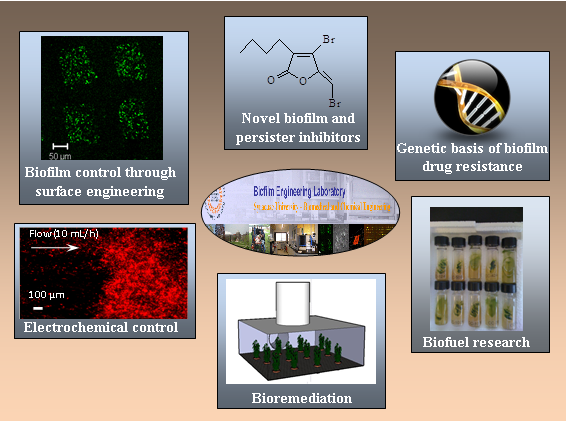
In the Biofilm Engineering Laboratory at Syracuse University, we have broad interests in biofilm research including genetic basis of multidrug resistance, biofilm control through surface engineering and bioelectric effects, development of novel biofilm and persister inhibitors, as well as biofilm engineering for biofuel production. We are intrigued by the opportunities in this field and welcome interested students and researchers to join us!
|
Overview of our research program
|